Exercise Induced Analgesia
Why does exercise make you feel good? The popular idea is that exercise gives you “endorphins,” and this explanation is actually not far from the mark. The word endorphin is short for endogenous morphine, which is an opioid “drug” that may start to flow when you move. In this post, I’ll provide a detailed discussion of various mechanisms for “exercise induced analgesia” including activation of the body’s pain inhibitory system. We need this system working well not just so we can get a runner’s high, but to help prevent chronic pain. Regular physical activity might be the best way to maintain its health and proper function.
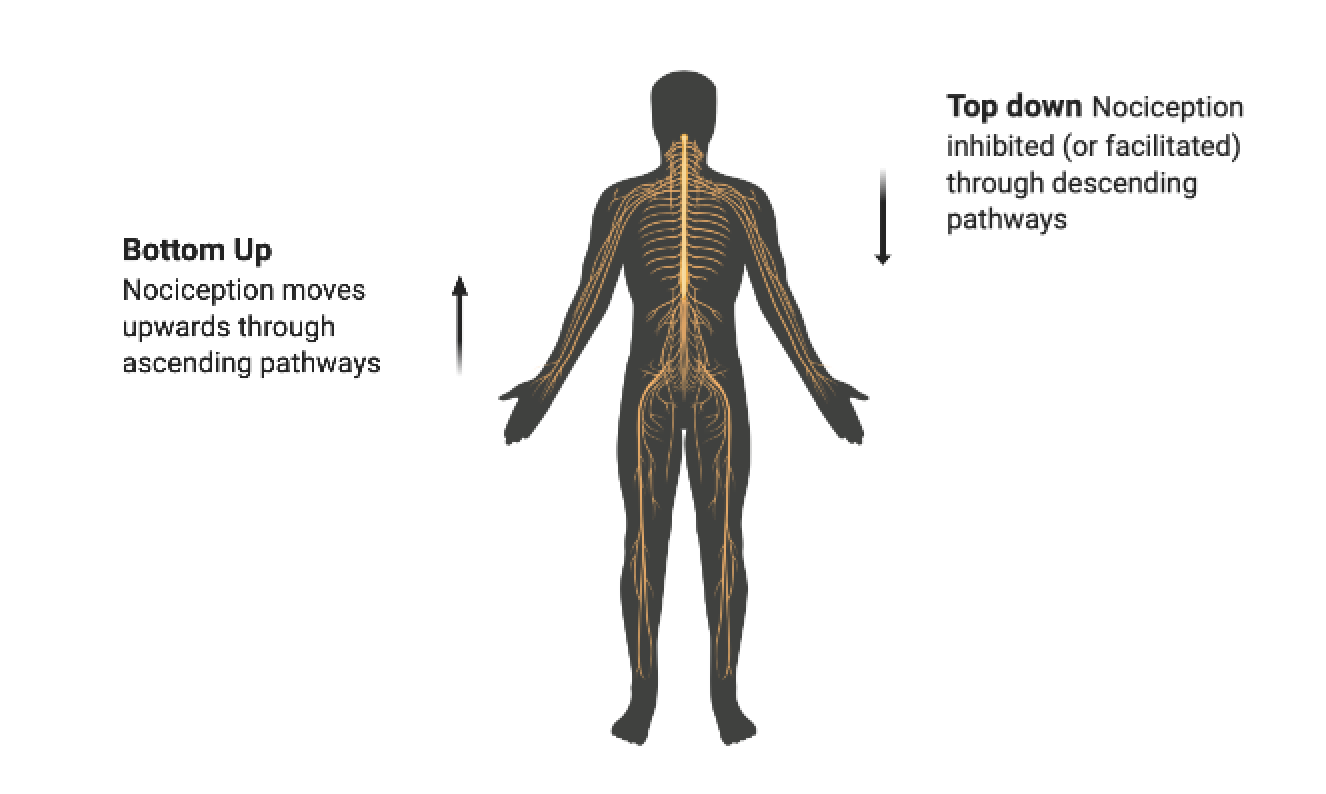
Top down control of pain: descending inhibition
One key mechanism for exercise induced analgesia is descending inhibition of nociception, which occurs when certain brain areas suppress nociceptive signals in the spinal cord. This is called “top-down” control over pain, because the brain has an active say in whether pain occurs, as opposed to passively reflecting bottom-up signals from the body.
For example, in an emergency, the brain might recognize that survival requires running, so it activates the descending inhibitory system to suppress nociception. (Interestingly, this suppression is selective, focused more on C fibers than fast acting A fibers, which means that “old news” about existing tissue damage is effectively tuned out, while the system remains alert to sensory information about new injuries (Heinricher 2010).
The descending inhibitory system is generally activated by vigorous physical activity. During a marathon (which may be perceived as a minor emergency), the feet and knees may generate a lot of nociception, but much of it will be inhibited if higher brain centers determine that completing the marathon is a valuable goal. Not surprisingly, triathletes have supercharged descending inhibitory systems: they truly get high from running. People with chronic pain and fibromyalgia are at the opposite end of the spectrum - their descending inhibitory systems do not work very well at all, which is why they often feel worse not better during physical activity. Many experts believe that the behavior of the descending inhibitory system is a critical factor in explaining chronic pain (Ossipov 2012, 2015).
Key anatomical structures involved in descending inhibition
The periaqueductal gray (PAG) was the first brain region shown to activate an endogenous pain inhibitory system, as its stimulation caused immediate pain relief (Kwon 2014). The PAG receives inputs from parts of the limbic system and brain areas involved in processing emotion, fear, and motivation. These connections are understood to be mechanisms by which thoughts and emotions can affect pain. For example, the PAG plays a role in the placebo response.
The PAG influences descending inhibition primarily through its connections to the rostral ventromedial medulla (RVM), which can also facilitate nociception. The decision about whether to facilitate or inhibit nociception is based on considerations by higher level brain areas about the meaning of the nociception and how to respond to it (Melzack and Wall 2014).
Just as suppression of pain could be advantageous in highly stressful or dangerous situations where other behaviors must pre-empt pain responses and recuperative behaviors in order to ensure survival, facilitation of pain could promote recuperative behaviors during illness, and enhance vigilance in situations where threat is possible, but not imminent.
(Heinricher 2009). Two types of neurons have been identified in the RVM as being responsible for pain modulation: on-cells and off-cells. Off-cells trigger descending inhibition, and on-cells create descending facilitation (Kwon 2014). The dynamic balance between on and off is dictated by behavioral priorities, fears, and other factors evaluated by higher structures in the brain (Heinricher 2009). It has been suggested that an imbalance toward facilitation may underlie pathological pain states (Ossipov 2012).
A primary target for descending modulation is the dorsal horn of the spine, which is the point where peripheral nerves connect to the spinal cord. The dorsal horn acts as a “gate” on nociception, because its sensitivity helps determine whether nociception moves from the body to the brain. Sensitivity is determined in part by ascending sensory information (the amount of nociception from the periphery), but also the descending modulation from the PAG-RVM system. Thus, inadequate inhibition can be an important cause of central sensitization and chronic pain states (Ossipov 2012).
There are a wide variety of chemical substances that act to inhibit nociception, including endogenous opioids, cannabinoids, serotonin, and catecholamines. For example, opiod peptides bind to opioid receptors on many parts of central and perisperhal nervous system, and this decreases the excitability of the nociceptors, causing them to fire less (Da Silva 2018).
Immune system changes
Physical activity can also affect pain by causing complex changes in the behavior of the immune system, both locally and globally (Petersen 2005; Sluka 2018). For example, exercise can modulate the phenotype of macrophages in muscle, making them more likely to release anti-inflammatory as opposed to pro-inflammatory cytokines. There is research indicating that regular exercise can reduce the level of circulating inflammatory cytokines in the bloodstream, in patients with fibromyalgia and healthy controls. Other research shows that regular exercise may reduce glial cell activation in the central nervous system, reduce inflammatory cytokines, and increase anti-inflammatory cytokines in the dorsal horn (Sluka 2018).
Conditioned pain modulation
Another reason exercise may kill pain is through conditioned pain modulation or “CPM” (also referred to as diffuse noxious inhibitory control or counter-irritation). CPM describes the phenomenon whereby “pain inhibits pain.”
CPM has been studied for at least 70 years, because it’s fairly easy to study. Experiments usually look something like this: (1) a person receives a noxious stimulus (such as pressure) and reports pain level, and then (2) the person is exposed to a painful “conditioning stimulus”, such as cold water immersion of the hand, and then (3) the person receives another round of the initial noxious stimulus and reports pain level. Usually, the second round will feel less painful, and the degree of pain relief is considered a measure of how well the descending inhibitory system is functioning.
Here are some interesting facts about CPM:
CPM is the likely mechanism for pain reduction in a wide variety of manual therapies, including deep tissue massage, acupuncture, dry needling, instrument assisted soft tissue manipulation, and foam rolling. If any of these treatments help with your pain, it is likely that you can get the same effect from the right kind of exercise.
CPM is less effective in patients with IBS, TMJ, tension headache, fibromyalgia and depression (Yarntisky 2010).
Pre-operative CPM efficacy predicts post-operative pain levels, including which patients transition from acute to chronic pain (Yarnitksy 2010).
CPM efficiency predicts the strength of exercise induced analgesia, and they probably rely on at least some common mechanisms (Stolzman 2016).
People who frequently engage in vigorous activity have enhanced CPM compared to less active people (Sluka 2016).
Can we improve descending inhibition through exercise?
We know that physical inactivity is a risk factor for chronic pain, that exercise stimulates the pain modulatory system, and that a healthy balance in the system is necessary for avoiding chronic pain. This raises the question of whether regular exercise is a way to maintain and recover the proper function of the pain inhibitory system. Sluka and colleagues propose that the answer is yes:
regular physical activity changes the state of central pain inhibitory pathways and the immune system to result in a protective effect against a peripheral insult.
The evidence in support of this contention is confusing and mixed, but there are some encouraging results. In addition to the research discussed above, it has been shown that regular aerobic exercise is an effective treatment for fibromyalgia, and can also increase tolerance to ischemic pain in healthy individuals (Sluka 2016; Ellingson 2016). On the other hand, it has been found that aerobic capacity does not predict pain level in response to a given stimulus, and several studies show that exercise can cause pain in fibromyalgia or lead to flareups (Ellingson 2016). In general, almost any kind of exercise seems to help with almost any kind of chronic pain, but the effect sizes tend to be small.
Closing thoughts
Exercised induced analgesia is not just about getting some temporary feel-good chemicals from a jog or weightlifting session. It is about tuning up a system whose proper function is necessary to keep you feeling good all the time.
A word of caution about the physiology discussed here: it’s very interesting to learn about all of the individual micro-level players in the descending inhibitory system, but we must remember that they interact in highly dynamic and complex ways. Therefore, their collective effect may be very hard to predict by analyzing the separate parts. For example, serotonin inhibits pain in some contexts but facilitates it in others. This is why therapies aimed at very specific targets (especially drug therapies) may have unintended effects, or even cause the opposite of the intended effect.
In my view, the more practical perspective is to keep in mind the purpose for which the descending inhibitory system evolved, which is to help you perform personally valued movements in the face of potential physical danger. Descending inhibition is there to keep you moving even when the movements are generating some nociception, especially when those movements are meaningful and intrinsically motivating. To keep the system healthy, challenge it to perform this function at a goldilocks level of intensity as often as possible, and see if it adapts to get better at its job.
This is how we improve the function of all the different bodily systems that help us move around, including muscles, tendons, bones, and the cardiovascular system. When they are put under an appropriate level of challenge or stress to do their jobs, they get better at doing them. Perhaps something similar holds true for the descending inhibitory system. Find movements that make you feel good, or that at least give you a “good pain,” and do them frequently.
References
Da Silva Santos R, Galdino G. Endogenous systems involved in exercise-induced analgesia. J Physiol Pharmacol. 2018;69(1):3-13. doi:10.26402/jpp.2018.1.01
Kwon M, Altin M, Duenas H, Alev L. The role of descending inhibitory pathways on chronic pain modulation and clinical implications. Pain Pract. 2014;14(7):656-667. doi:10.1111/papr.12145
M.M. Heinricher, Tavares I, Leith JL, Lumb BM. Descending control of nociception. 2010;60(1):214-225. doi:10.1016/j.brainresrev.2008.12.009.Descending
Ossipov, Morimura. Descending pain modulation and chronicification of pain. Curr Opin Support Palliat Care. 2015;9(1):38-39. doi:10.1097/SPC.0000000000000055
Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154-1162. doi:10.1152/japplphysiol.00164.2004
Polaski AM, Phelps AL, Kostek MC, Szucs KA, Kolber BJ. Exercise-induced hypoalgesia: A meta-analysis of exercise dosing for the treatment of chronic pain. PLoS One. 2019;14(1):1-29. doi:10.1371/journal.pone.021041
Price TJ, Ray PR. Recent advances toward understanding the mysteries of the acute to chronic pain transition. Curr Opin Physiol. 2019;11:42-50. doi:10.1016/J.COPHYS.2019.05.015
Sluka KA, Frey-Law L, Hoeger Bement M. Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain. 2018;159(9):S91-S97. doi:10.1097/j.pain.0000000000001235
Ellingson LD, Stegner AJ, Schwabacher IJ, Koltyn KF, Cook DB. Exercise strengthens central nervous system modulation of pain in fibromyalgia. Brain Sci. 2016;6(1):13. doi:10.3390/brainsci6010008
Melzack and Wall. Textbook of Pain Ed. 6.
Zhuo M. Descending facilitation: From basic science to the treatment of chronic pain. Mol Pain. 2017;13:1-12. doi:10.1177/1744806917699212
Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23(5):611-615. doi:10.1097/ACO.0b013e32833c348b
Alsouhibani A, Vaegter HB, Bement MH. Systemic exercise-induced hypoalgesia following isometric exercise reduces conditioned pain modulation. Pain Med (United States). 2019;20(1):180-190. doi:10.1093/pm/pny057
Stolzman S, Bement M. Does exercise decrease pain via conditioned pain modulation in adolescents?". Pediatr Phys Ther. 2016;28(4):474. doi:10.1097/PEP.0000000000000313
Ossipov MH. The Perception and Endogenous Modulation of Pain. Scientifica (Cairo). 2012;2012:1-25. doi:10.6064/2012/561761
Yamamotová A. Mechanisms of exercise-induced hypoalgesia. Psychiatrie. 2018;22(1):33-38. doi:10.1016/j.jpain.2014.09.006.Mechanisms